Is Semaglutide The Same As Ozempic? Key Differences Explained
If you’re considering starting weight loss medication you might have heard the names Ozempic and semaglutide used interchangeably. In this article we’ll unpack the key similarities and differences so that you find the right weight loss medication for you.
If you’re considering starting weight loss medication you might have heard the names Ozempic and semaglutide used interchangeably. In this article we’ll unpack the key similarities and differences so that you find the right weight loss medication for you.
What is semaglutide?
Definition and classification
Semaglutide is the active ingredient in popular medications used to treat type 2 diabetes and obesity, such as Ozempic and Wegovy. These medications are classified as GLP-1s, or glucagon-like peptide-1, receptor agonists.
How does semaglutide work?
Semaglutide mimics the action of a naturally occurring hormone in the body called GLP-1. By targeting GLP-1 receptors throughout the body, semaglutide works to increase insulin secretion from the pancreas in response to eating, lowering blood sugar levels.
It also works on the region of the brain (hypothalamus) involved in appetite regulation, by signalling a feeling of fullness and reducing appetite. Semaglutide also slows down the rate at which food leaves your stomach, helping you to feel fuller for longer.
Understanding Ozempic
What is Ozempic?
Ozempic is a GLP-1 medication that contains semaglutide as its active ingredient. It was approved by the FDA in 2017 for the treatment of type 2 diabetes, as its use in clinical trials saw clinically significant data that transformed the landscape of type 2 diabetes treatments. [1]
Primary uses of Ozempic
Currently, Ozempic is licensed to treat type 2 diabetes alongside changes to diet and exercise. [2] It is also approved to help adults with type 2 diabetes who have heart disease by lowering the risk of serious heart problems. While Ozempic is licensed to primarily treat type 2 diabetes, it can be prescribed off-label for weight loss at the discretion of your physician.*
Semaglutide vs. Ozempic: Key differences
Active ingredients and formulations
To put it simply, Ozempic is the branded name used by Novo Nordisk for semaglutide to treat type 2 diabetes. In other words, semaglutide is the active ingredient in Ozempic.
Approved uses and indications
Ozempic is officially approved for the treatment of type 2 diabetes and reducing cardiovascular risks. Semaglutide is also present in mediations such as Wegovy, which is approved for the treatment of obesity. [3]
Dosage and administration guidelines
Ozempic treatment starts at the low dose of 0.25 mg. The dose of Ozempic is administered once weekly and gradually increases every 4 weeks. In theory, this should keep side effects to a minimum, as your body becomes adjusted to the medication.
- Weeks 1-4: 0.25 mg once weekly
- Weeks 5-8: 0.5 mg once weekly
- Weeks 9-12: 1 mg once weekly
- Weeks 13-16: 2 mg once weekly (maintenance dose)
The maximum dosage of Ozempic is 2 mg, which you may begin at 13 weeks of treatment. The 0.5 mg dose may be sufficient in helping people achieve their blood sugar goals, and there may not be a need in increasing your dose from this amount.
Ozempic is administered as a subcutaneous (under the skin) injection, directly into the layer of fatty tissue beneath your skin. This can include areas such as your stomach, thigh or back of the arm. It is recommended to rotate the site of your weekly injection to prevent any skin irritation or scar tissue build-up.
Semaglutide and Weight Loss
Efficacy for weight management
Semaglutide is also the active ingredient in Wegovy and has been shown to be highly effective for the treatment of obesity. Clinical trials have reported an average body weight reduction of 14.9% after 68 weeks of once-weekly 2.4 mg semaglutide treatment. [4]
As a result, Wegovy was approved for the treatment of obesity by the FDA in 2021 [3], in addition to a reduced calorie diet and increased physical activity. Patients eligible for Wegovy must have a BMI of 30 or over, or 27 or over in the presence of at least one other weight-related health condition (for example prediabetes, high blood pressure, or high cholesterol).
Comparison with other weight loss medications
Semaglutide has demonstrated superior weight loss outcomes compared to other available medications on the market. After 2 years of 2.4 mg once-weekly semaglutide treatment, average weight loss was -15.2%. [5] The same study reported that 77.1% of semaglutide-treated participants lost ≥5% of baseline body weight and 44.2% achieved ≥10% weight loss after 2 years.
Other weight loss medications, including Orlistat which limits fat absorption in the gut, do not result in weight loss at the same success rate. Clinical trials have shown the percentage of participants achieving ≥10% weight loss to be 26.2% after 4 years. [6]
Understanding Wegovy
What is Wegovy?
Wegovy is the brand name used by Novo Nordisk for semaglutide for the treatment of obesity.
Distinctions between Wegovy and Ozempic
While both Wegovy and Ozempic both contain the same active ingredient - semaglutide - they are available in different doses.
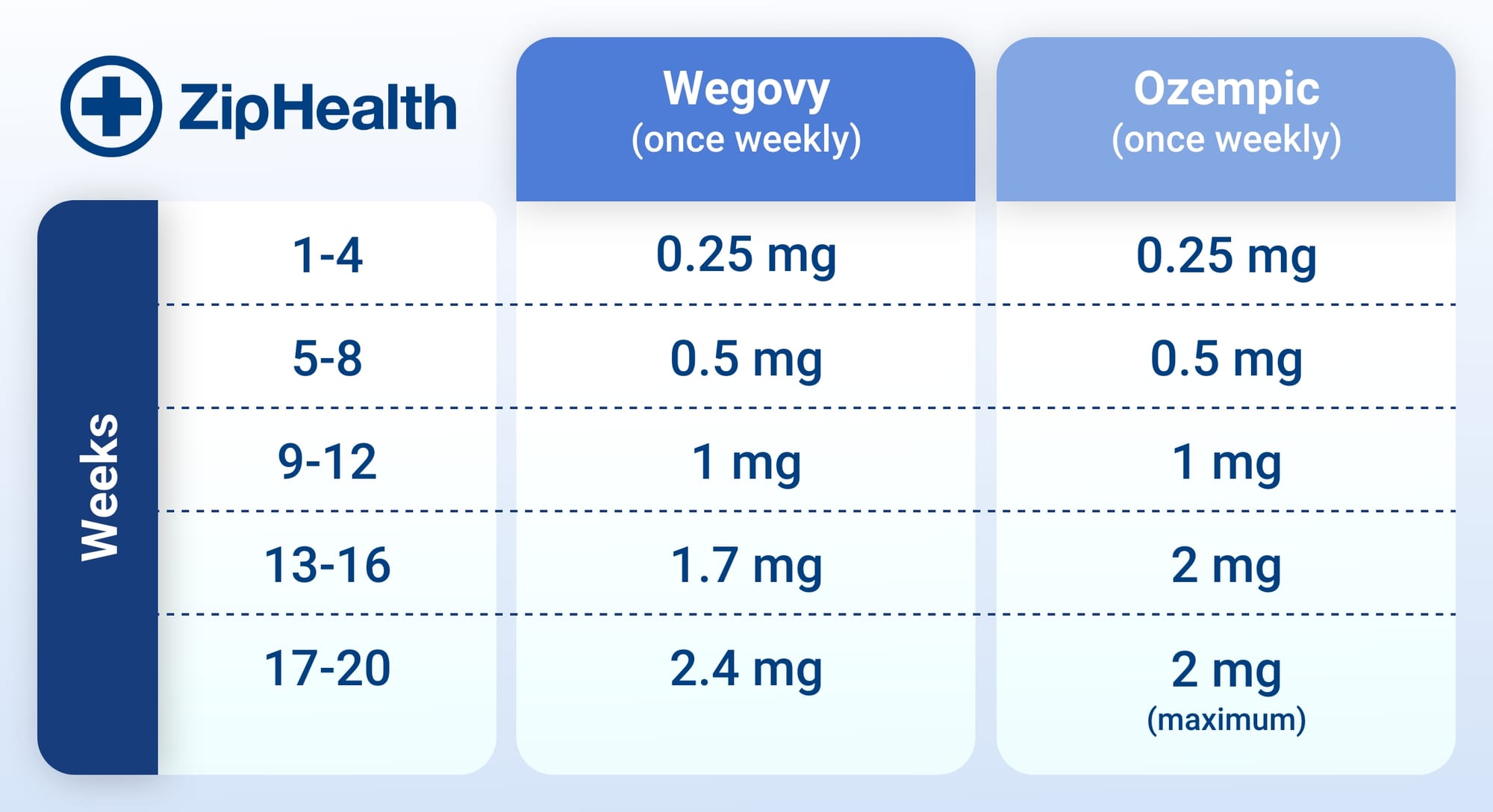
Just like with Ozempic, your dosage for Wegovy will also increase every 4 weeks. 2.4 mg is the maximum dosage of Wegovy which you begin at 17 weeks of your weight-loss treatment plan.
The maximum dose for Wegovy is higher than Ozempic due to the different conditions both medications treat - a higher dose of semaglutide was proven to be highly effective in aiding weight loss. Meanwhile, lower doses can help patients with type 2 diabetes effectively manage their blood sugar levels.
Compounded Semaglutide
What is compounded semaglutide?
Compounded Semaglutide** is a non-branded version of semaglutide which contains the exact same active ingredient as Wegovy, making it equally effective for achieving substantial weight loss. While permitted under federal law, it’s important to note that compounded semaglutide is not FDA-approved and does not undergo FDA safety, effectiveness, or quality reviews.
However, it is produced by specialized pharmacies that adhere to rigorous testing and strict safety protocols to ensure the highest quality standards. This process results in a high-quality product with a proven track record of consistency and medical effectiveness. An additional benefit of Compounded Semaglutide is its cost-effectiveness, often being up to 80% cheaper than branded alternatives.
Despite the lack of FDA approval, the specialized manufacturing process and adherence to safety standards aim to provide a safe and effective alternative for patients seeking semaglutide treatment.
Availability and usage considerations
Compounded Semaglutide** treatment begins with a low dose which gradually increases every four weeks to ensure optimal results and minimize side effects, just like with the branded-alternatives.
When starting Compounded Semaglutide**, the initial dose is 0.25 mg, injected once a week for the first four weeks. After this period, the dosage will be increased every four weeks, as tolerated.
If you miss a dose of Compounded Semaglutide**, your healthcare provider will advise you when to take your next dose or if you should skip the missed dose and begin again on the next scheduled day.
Safety and side effects
Common side effects of semaglutide and Ozempic
The most common side effects of semaglutide both branded and unbranded have been reported to be gastrointestinal issues, with 43.9% of patients on 2.4 mg semaglutide reported to have experienced nausea, 29.7% experienced diarrhea, 24.5% experienced vomiting, and 24.2% experienced constipation. [7]
Potential long-term risks
More severe side effects include pancreatitis, which is inflammation of the pancreas, and thyroid cancer.
Pancreatitis has been noted in clinical trials, and symptoms to look out for include persistent and severe abdominal pain, which sometimes radiates to the back, which may or may not be accompanied with vomiting. [3] It is important that semaglutide treatment is stopped if pancreatitis is suspected.
Studies of semaglutide treatment in rodents have noted the formation of thyroid C-cell tumours. While this effect is not yet known in humans, semaglutide-containing medications come with a boxed warning and cannot be prescribed to patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with multiple endocrine neoplasia syndrome type 2 (MEN2). [2]
Want to try medicated weight loss?
Our clinicians assess your medical history and help you decide whether our treatment can help you on your weight loss journey. Start an online consultation today.
*Ozempic is FDA-approved for type 2 diabetes, but may be prescribed off-label for obesity at the discretion of the prescribing physician.
**Compounded drugs are permitted to be prescribed under federal law but are not FDA-approved and do not undergo FDA safety, effectiveness, or quality review.
References
[1] Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, Bain SC. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017 Apr;5(4):251-260. doi: 10.1016/S2213-8587(17)30013-X. Epub 2017 Jan 17. PMID: 28110911.
[2] HIGHLIGHTS OF PRESCRIBING INFORMATION [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/209637s020s021lbl.pdf
[3] Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Mar 18;384(11):989-1002. doi: 10.1056/NEJMoa2032183. Epub 2021 Feb 10. PMID: 33567185.
[4] Garvey, W.T., Batterham, R.L., Bhatta, M. et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med 28, 2083–2091 (2022). https://doi.org/10.1038/s41591-022-02026-4
[5] Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004 Jan;27(1):155-61. doi: 10.2337/diacare.27.1.155. Erratum in: Diabetes Care. 2004 Mar;27(3):856. PMID: 14693982.
[6] Wharton S, Calanna S, Davies M, Dicker D, Goldman B, Lingvay I, Mosenzon O, Rubino DM, Thomsen M, Wadden TA, Pedersen SD. Gastrointestinal tolerability of once-weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss. Diabetes Obes Metab. 2022 Jan;24(1):94-105. doi: 10.1111/dom.14551. Epub 2021 Oct 4. PMID: 34514682; PMCID: PMC9293236.
[7] Wharton S, Calanna S, Davies M, Dicker D, Goldman B, Lingvay I, Mosenzon O, Rubino DM, Thomsen M, Wadden TA, Pedersen SD. Gastrointestinal tolerability of once-weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss. Diabetes Obes Metab. 2022 Jan;24(1):94-105. doi: 10.1111/dom.14551. Epub 2021 Oct 4. PMID: 34514682; PMCID: PMC9293236.
